Alloys are essential for buildings, transportation, appliances, and tools. They’ve also caused engineers to face a common materials trade-off: hard alloys tend to be brittle and break under strain, but if flexible under strain, they tend to dent easily.
Twenty years ago, researchers developed medium- and high-entropy alloys, stable materials that combine hardness and flexibility in a way that conventional alloys do not. A UCLA-led research team has provided a view of the structure and characteristics of medium- and high-entropy alloys. Using advanced imaging, the team mapped, for the first time ever, the 3-D atomic coordinates of such alloys and correlated the mixture of elements with structural defects.
The study was published Dec. 20 in the journal Nature.
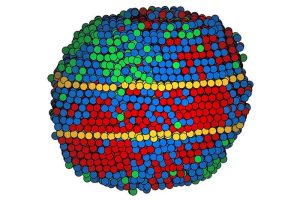
Credit: Miao Lab/UCLA
Medium-entropy alloys combine three or four metals in nearly equal amounts; high-entropy alloys combine five or more similarly. Conventional alloys are mostly one metal, with others intermixed in lower proportions.
The colleagues focused on a type of structural defect called a twin boundary, a key factor in medium- and high-entropy alloys’ unique combination of toughness and flexibility. Twinning happens when strain causes one section of a crystal matrix to bend diagonally. At the same time, the atoms around it remain in their original configuration, forming mirror images on either side of the boundary.
Researchers used an array of metals to make nanoparticles so small they can be measured in billionths of a meter. Six medium-entropy alloy nanoparticles combined nickel, palladium, and platinum. Four nanoparticles of a high-entropy alloy combined cobalt, nickel, ruthenium, rhodium, palladium, silver, iridium, and platinum.
The scientists liquified the metal at over 2,000 degrees Fahrenheit for five-hundredths of a second, then cooled it down in less than one-tenth that time. The idea is to fix the solid alloy in the same varied mixture of elements as a liquid. The shock of the process induced twin boundaries in six of the 10 nanoparticles; four of them each had a pair of twins.
The scientists mapped each atom in the medium-entropy alloy nanoparticles. Some of the metals in the high-entropy alloy were too similar in size for electron microscopy to differentiate among them. So, the map of those nanoparticles grouped the atoms into three categories.
The researchers observed that the more atoms of different elements are mixed, the more likely the alloy’s structure will change in a way that contributes to matching toughness with flexibility.

