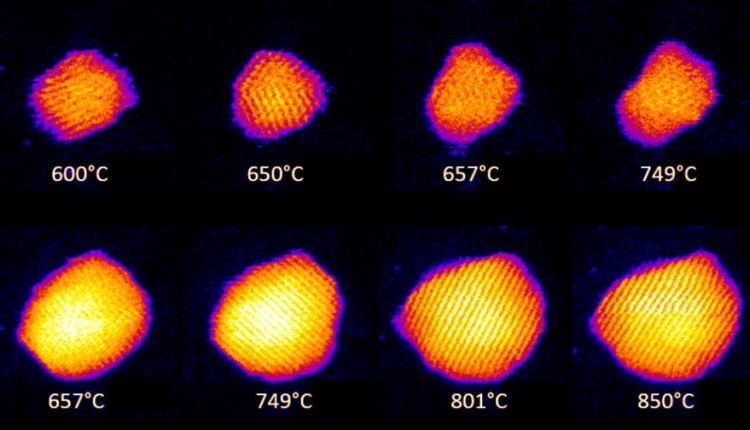
How nanoparticles melt has been a mystery since the study of the extremely small began. While initial theoretical models describing melting have been around for a century, with more relevant models being about 50 years old, the way melting behavior changed on the nanoscale has been an open question.
Professor Richard Palmer, who led a Swansea University College of Engineering team, to study how gold nanoparticles melt, said of research recently published in Nature Communications, said, “Given that the theoretical models are now rather old, there was a clear case for us to carry out our new imaging experiments to see if we could test and improve these theoretical models.”
The research team used gold in their experiments as it acts as a model system for noble and other metals. The team arrived at their results by imaging gold nanoparticles, with diameters ranging from 2 to 5 nanometres, via aberration-corrected scanning transmission electron microscope. Their observations were later supported by large-scale quantum mechanical simulations.
Professor Palmer said: “We were able to prove the dependence of the melting point of the nanoparticles on their size and for the first time see directly the formation of a liquid shell around a solid core in the nanoparticles over a wide region of elevated temperatures, in fact for hundreds of degrees.
“This helps us to describe accurately how nanoparticles melt and to predict their behavior at elevated temperatures. This is a science breakthrough in a field we can all relate to—melting—and will also help those producing nanotech devices for a range of practical and everyday uses, including medicine, catalysis and electronics.”
Source: Swansea University
