Solvent Reorganization Energy Can Be Used to Select Electrolytes for Metal-Air Batteries
By Ruth Seeley
As we continue to demand more of our batteries—smaller, cheaper, and yet with greater capacity—the limitations of lithium-ion become more apparent and scientists continue to try to find alternatives that will do everything lithium-ion can and more.
As successors to lithium-ion, scientists have been looking at metal-air batteries because of their exceptional gravimetric energy densities that could potentially enable electric cars to travel a thousand miles or more on a single charge.
A promising new member of the alkali-metal-air battery family is the potassium-air battery, which has more than three times the theoretical gravimetric energy density of lithium-ion batteries. A key challenge in designing potassium-air batteries is choosing the right electrolyte, the liquid which facilitates the transfer of ions between the cathode and anode.
Typically, electrolytes are chosen using a trial-and-error approach based on rules of thumb correlating several electrolyte properties, followed by exhaustive (and time-consuming) testing of several electrolyte candidates to see if the desired performance is achieved.
Now researchers from Washington University in St. Louis, led by Vijay Ramani at the McKelvey School of Engineering, have shown how electrolytes for alkali-metal air batteries can be chosen in a breakthrough advance that shows how a single, easy-to-measure parameter descriptor of the solvation energy correlates with both ion transport and surface reaction kinetics. This will allow the rational development of new high-performance electrolytes for metal-air batteries.
Ramani’s team studied the fundamental interactions between the salt and solvent in the electrolyte and show how these interactions can influence overall battery performance. They developed a novel parameter, namely the “Electrochemical” Thiele Modulus, a measure of the ease of ion transport to and reaction at an electrode surface.
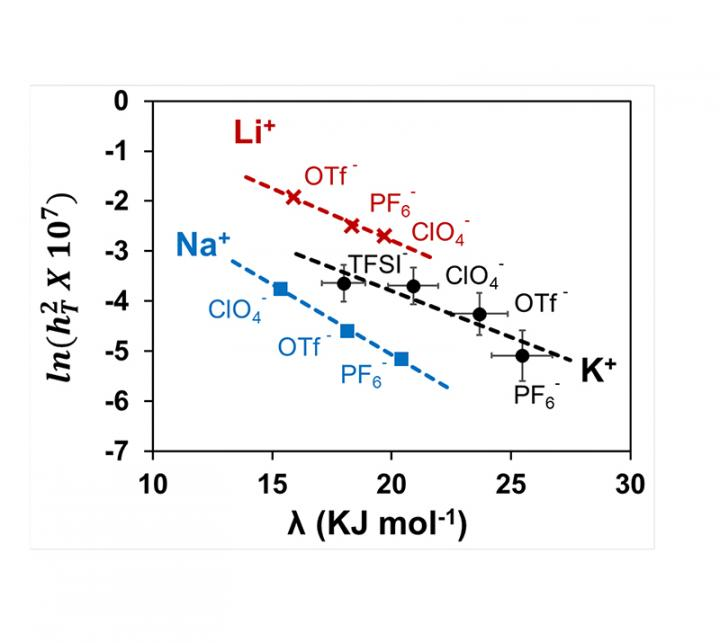
This research documents the first time that the Nobel Prize-winning Marcus-Hush theory of electron transfer has been used to study the impact of electrolyte composition on the movement of ions through the electrolyte, and their reaction at the surface of the electrode.
This Thiele Modulus was shown to exponentially decrease with increasing solvent reorganization energy—a measure of the energy needed to modify the solvation sphere of a dissolved species. Thus, the solvent reorganization energy could be used to rationally select electrolytes for high-performance metal-air batteries without trial-and-error.
“We started out trying to better understand the influence of the electrolyte on the oxygen reduction reaction in metal-air battery systems,” said Shrihari Sankarasubramanian, a research scientist on Ramani’s team and lead author of the study. “We ended up showing how the diffusion of ions in the electrolyte and the reaction of these ions on the electrode surface are both correlated to the energy needed to break the solvation shell around the dissolved ions.”