We use lightweight, high-capacity lithium-ion batteries in our personal electronics. These conventional lithium-ion batteries use organic electrolytes that are highly flammable and have caused fires or explosions.
Researchers are developing aqueous electrolyte batteries as promising replacements, but so far, zinc-ion batters are impractical due to the inferior reversibility of the zinc anode.
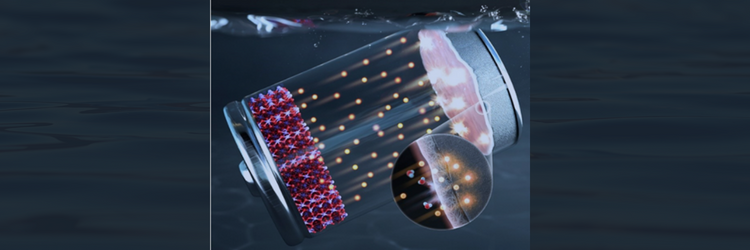
Researchers at POSTECH are aiming to fix that. They’ve developed a stable aqueous zinc-ion battery that uses water as an electrolyte. It features a protective polymer layer that prevents electrode corrosion, increasing zinc anode stability and the electrochemical stability of the aqueous zinc-ion battery.
The POSTECH research team coated its zinc anode with a multifunctional protective layer using a block copolymer. The polymer layer is elastic and stretchable, enduring volume expansion during battery charging and discharging. And most importantly, it contributes to a long-term zinc anode lifespan. It also improves electrode stability by suppressing unnecessary chemical/electrochemical reactions in the electrolyte on the electrode surface.
They published their work in Cell Reports Physical Science.