Bendy Energy Storage Breakthrough for Portable Power Supplies
High-powered, fast-charging supercapacitors are usually not capable of holding a large amount of energy in a small space. But a new supercapacitor developed at University College London that uses innovative graphene electrode material with pores that can be changed in size to store the charge more efficiently and has both high power and energy densities.
While at the proof-of-concept stage, it shows enormous potential as a portable power supply in several practical applications including electric vehicles, phones and wearable technology.
“Our new supercapacitor is extremely promising for next-generation energy storage technology as either a replacement for current battery technology, or for use alongside it, to provide the user with more power,” said first author Dr. Zhuangnan Li (UCL Chemistry).
“We designed materials which would give our supercapacitor a high power density — that is how fast it can charge or discharge — and a high energy density — which will determine how long it can run for. Normally, you can only have one of these characteristics but our supercapacitor provides both, which is a critical breakthrough.
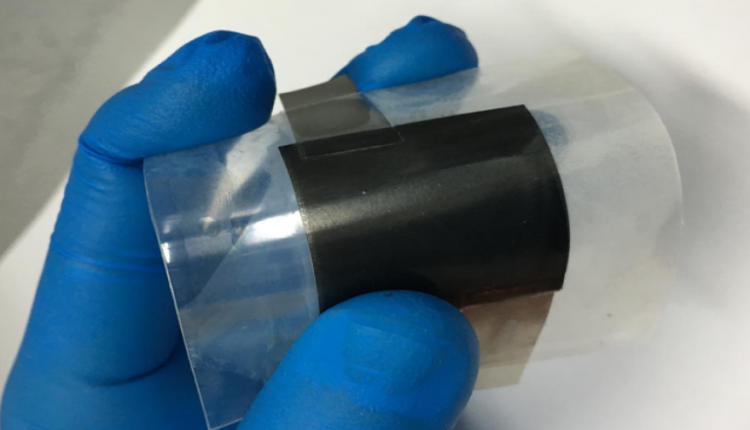
“Moreover, the supercapacitor can bend to 180 degrees without affecting performance and doesn’t use a liquid electrolyte, which minimizes any risk of explosion and makes it perfect for integrating into bendy phones or wearable electronics.”
A team of chemists, engineers and physicists worked on the new design, whose tuning maximizes the energy density of the supercapacitor to a record 88.1 Watt-hour per litre (Wh/L), which is the highest ever reported energy density for carbon-based supercapacitors.
Similar fast-charging commercial technology has a relatively poor energy density of 5-8 Wh/L and traditional slow-charging but long-running lead-acid batteries used in electric vehicles typically have 50-90 Wh/L.
While the supercapacitor developed by the team has a comparable energy density to state-of-the-art value of lead-acid batteries, its power density is two orders of magnitude higher at over 10,000 Wh/L.
“Successfully storing a huge amount of energy safely in a compact system is a significant step towards improved energy storage technology. We have shown it charges quickly, we can control its output and it has excellent durability and flexibility, making it ideal for development for use in miniaturized electronics and electric vehicles. Imagine needing only 10 minutes to fully charge your electric car or a couple of minutes for your phone and it lasting all day,” said Professor Ivan Parkin, Dean of UCL Mathematical & Physical Sciences.
The researchers made electrodes from multiple layers of graphene, creating a dense but porous material capable of trapping charged ions of different sizes. They characterized it using a range of techniques and found it performed best when the pore sizes matched the diameter of the ions in the electrolyte.
The optimized material, which forms a thin film, was used to build a proof-of-concept device with both a high power and high energy density.
The 6cm x 6cm supercapacitor was made from two identical electrodes layered either side of a gel-like substance which acted as a chemical medium for the transfer of electrical charge. This was used to power dozens of light-emitting diodes (LEDs) and was found to be highly robust, flexible and stable.
Even when bent at 180 degrees, it performed almost the same as when it was flat, and after 5,000 cycles, it retained 97.8% of its capacity.
Source: University College London
