Cold weather is the enemy of our current batteries, slowing the chemical reaction inside, making them less reliable and negatively affecting their performance.
In the search for a reliable, quick-charging, cold-weather battery for automobiles, a self-assembling, thin layer of electrochemically active molecules may be the solution, according to a team of researchers.
“The lithium metal battery is the next generation of battery after the lithium ion battery,” said Donghai Wang, professor of mechanical engineering and a key researcher in the Battery and Energy Storage Technology Center, Penn State. “It uses a lithium anode and has higher energy density, but has problems with dendritic growth, low efficiency and low cycle life.”
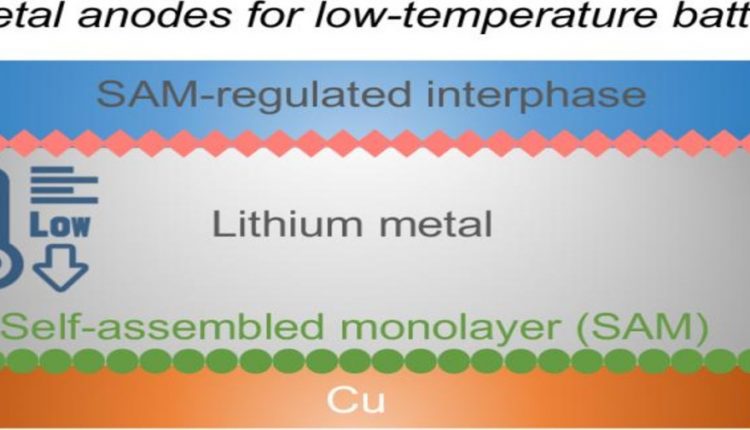
The solution to these problems, according to the researchers, is a self-assembling monolayer that is electrochemically active so that it can decompose into its proper components and protect the surface of the lithium anode.
The battery is composed of the lithium anode, a lithium metal oxide cathode and an electrolyte which also has lithium-ion conducting materials and the protective, thin film layer. Without this layer, the battery would tend to grow lithium crystal spikes if charged rapidly or under cold conditions. These lithium spikes eventually short out the battery, greatly decreasing the usefulness and cycle life.
“The key is to tune the molecular chemistry to self-assemble on the surface,” said Wang. “The monolayer will provide a good solid electrolyte interface when charging, and protect the lithium anode.”
The researchers deposit the monolayer on a thin copper layer. When the battery charges, lithium hits the monolayer and decomposes to form a stable interfacial layer. Some lithium is deposited on the copper along with the remaining layer, and the decomposed portion of the original layer reforms on top of the lithium, protecting the lithium and preventing dendrites of lithium from forming.
According to the researchers, this technology can increase the amount of storage capacity of the battery and can increase the number of times the battery can be charged. However, at this point, the battery can only be charged a few hundred times. The researchers reported their work in a recent issue of Nature Energy.
“The key is that this technology shows an ability to form a layer when needed on time and decompose and spontaneously reform so it will stay on the copper and also cover the surface of the lithium,” said Wang. “Eventually it could be used for drones, cars, or some very small batteries used for underwater applications at low temperatures.”
Source: Penn State
