Researchers at Oregon State University are demonstrating that iron, instead of cobalt and nickel, can be used as a cathode material in lithium-ion batteries. They published their findings in Science Advances.
So far, the cathode represents 50% of the cost of making a lithium-ion battery cell, but cost isn’t the only advantage iron provides. Iron-based cathodes also allow for greater safety and sustainability, as well.
As we manufacture more lithium-ion batteries, the global demand for nickel and cobalt has soared. Eventually, this will cause shortages in nickel and cobalt, putting the brakes on battery production. The energy density of these elements is at its ceiling level. Pushed further, oxygen released during charging could cause batteries to ignite. Cobalt is toxic and can contaminate ecosystems and water sources if it leaches out of landfills.
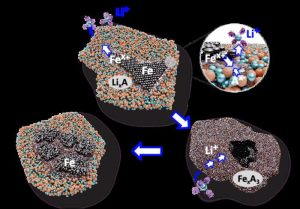
As its name suggests, a lithium-ion battery charges via lithium ions moving through the electrolyte from the anode to the cathode during discharge and back during recharging. The research team increased the reactivity of iron in their cathode by designing a chemical environment based on a blend of fluorine and phosphate anions—negatively charged ions. Mixed as a solid solution, this allows for the reversible conversion of a fine mixture of iron powder, lithium fluoride, and lithium phosphate into iron salts.
Xiulei “David” Ji, a researcher at Oregon State, said that they must improve storage efficiency. So far, not all of the electricity put into the battery during charging is available for use upon discharge. When those improvements are made, the battery will cost less and be more sustainable.

