Dust-sized sensors can be implanted for real-time organ monitoring
Engineers from the University of California, Berkeley have developed the first dust-sized, wireless sensors that can be implanted in the body. The breakthrough brings us one step closer the a day we can monitor internal nerves, muscles or organs in real time.
And, since these batteryless sensors could also be used to stimulate nerves and muscles, the technology also paves the way for “electroceuticals” capable of treating disorders such as epilepsy or stimulation of the immune system.
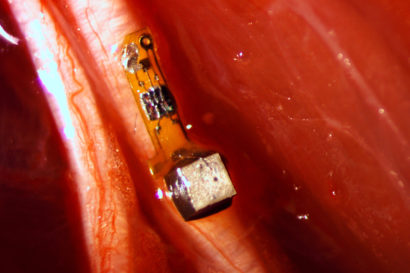
The “neural dust”, as its being referred to, was implanted in the muscles and peripheral nerves of rats. It is unique in that ultrasound is used both to power and read out the measurements. Ultrasound technology is already well-developed for hospital use, and ultrasound vibrations can penetrate nearly anywhere in the body, unlike radio waves.
“I think the long-term prospects for neural dust are not only within nerves and the brain, but much broader,“ said Michel Maharbiz, an associate professor of electrical engineering and computer sciences and one of the study’s two main authors. “Having access to in-body telemetry has never been possible because there has been no way to put something supertiny superdeep. But now I can take a speck of nothing and park it next to a nerve or organ, your GI tract or a muscle, and read out the data.
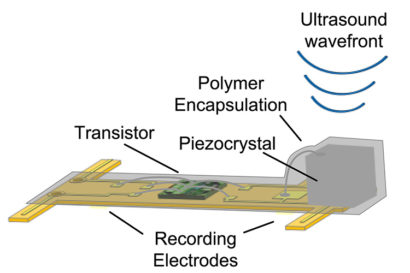
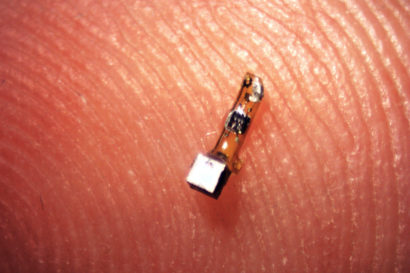
The 3-millimeter-long sensors have already shrunk to a 1 millimeter cube – about the size of a large grain of sand – and contain a piezoelectric crystal that converts ultrasound vibrations from outside the body into electricity to power a tiny, on-board transistor that is in contact with a nerve or muscle fiber. When there’s a voltage spike in the fiber, the circuit and the vibration of the crystal is altered, which changes the echo detected by the ultrasound receiver, typically the same device that generates the vibrations. This slight change, referred to as backscatter, allows them to determine the voltage.
In its experiments, the team powered up the passive sensors every 100 microseconds with six 540-nanosecond ultrasound pulses, which gave them a continual, real-time readout. They coated the first-generation motes – 3 millimeters long, 1 millimeter high and 4/5 millimeter thick – with surgical-grade epoxy, and they are now trying to build motes from biocompatible thin films which would potentially last in the body without degradation for a decade or more.
So far the researchers have conducted tests that involved the peripheral nervous system and muscles, but they say that neural dust motes could work equally well in the central nervous system and brain to control prostheses.
Current implantable electrodes degrade within two years and have to be connected to wires that pass through holes in the skull. With the new technology, wireless sensors could be sealed in, avoiding infection and unwanted movement of the electrodes.
“The original goal of the neural dust project was to imagine the next generation of brain-machine interfaces, and to make it a viable clinical technology,” said Ryan Neely, neuroscience graduate student . “If a paraplegic wants to control a computer or a robotic arm, you would just implant this electrode in the brain and it would last essentially a lifetime.”
The team is working to miniaturize the device further, as well as find more biocompatible materials and improve the surface transceiver that sends and receives the ultrasounds.
The researchers are also building little backpacks for rats to hold the ultrasound transceiver that will record data from implanted motes.
According to the researchers, with a reliable and minimally invasive neural pickup within the body, the technology could become the driver for a plethora of applications, things that don’t even exist yet.
Story via UC Berkeley.

Comments are closed, but trackbacks and pingbacks are open.