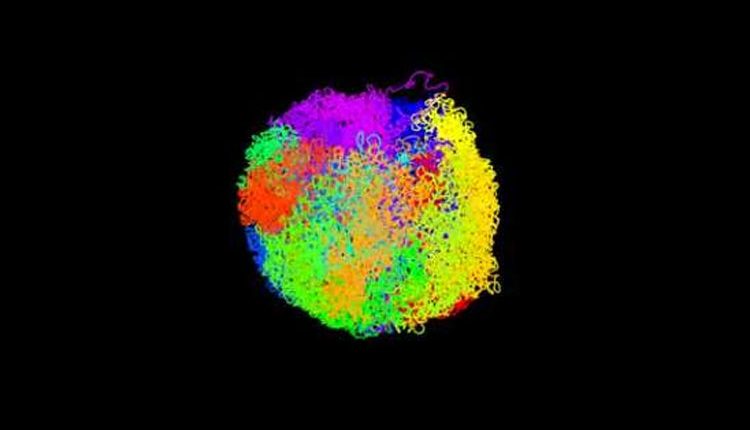
Showing how the DNA from all the chromosomes intricately folds to fit together inside the cell nuclei, Scientists have determined the first 3D structures of intact mammalian genomes from individual cells.
Researchers from the University of Cambridge and the MRC Laboratory of Molecular Biology used a combination of imaging and up to 100,000 measurements of where different parts of the DNA are close to each other to examine the genome in a mouse embryonic stem cell. Stem cells are ‘master cells’, which can develop – or ‘differentiate’ – into almost any type of cell within the body.
Most people are familiar with the well-known ‘X’ shape of chromosomes, but in fact chromosomes only take on this shape when the cell divides. Using their new approach, the researchers have now been able to determine the structures of active chromosomes inside the cell, and how they interact with each other to form an intact genome. This is important because knowledge of the way DNA folds inside the cell allows scientists to study how specific genes, and the DNA regions that control them, interact with each other. The genome’s structure controls when and how strongly genes – particular regions of the DNA – are switched ‘on’ or ‘off’. This plays a critical role in the development of organisms and also, when it goes awry, in disease.
The researchers have illustrated the structure in accompanying videos, which show the intact genome from one particular mouse embryonic stem cell. In the film, above, each of the cell’s 20 chromosomes is coloured differently.
In a second video regions of the chromosomes where genes are active are coloured blue, and the regions that interact with the nuclear lamina (a dense fibrillar network inside the nucleus) are coloured yellow. The structure shows that the genome is arranged such that the most active genetic regions are on the interior and separated in space from the less active regions that associate with the nuclear lamina. The consistent segregation of these regions, in the same way in every cell, suggests that these processes could drive chromosome and genome folding and thus regulate important cellular events such as DNA replication and cell division.
Professor Ernest Laue, whose group at Cambridge’s Department of Biochemistry developed the approach, commented: “Knowing where all the genes and control elements are at a given moment will help us understand the molecular mechanisms that control and maintain their expression.
“In the future, we’ll be able to study how this changes as stem cells differentiate and how decisions are made in individual developing stem cells. Until now, we’ve only been able to look at groups, or ‘populations’, of these cells and so have been unable to see individual differences, at least from the outside. Currently, these mechanisms are poorly understood and understanding them may be key to realising the potential of stem cells in medicine.”
The research, by scientists at the Departments of Biochemistry, Chemistry and the Wellcome-MRC Stem Cell Institute at the University of Cambridge, together with colleagues at the MRC Laboratory of Molecular Biology, is published today in the journal Nature.
Dr Tom Collins from Wellcome’s Genetics and Molecular Sciences team said: “Visualising a genome in 3D at such an unprecedented level of detail is an exciting step forward in research and one that has been many years in the making. This detail will reveal some of the underlying principles that govern the organisation of our genomes – for example how chromosomes interact or how structure can influence whether genes are switched on or off. If we can apply this method to cells with abnormal genomes, such as cancer cells, we may be able to better understand what exactly goes wrong to cause disease, and how we could develop solutions to correct this.”

Comments are closed, but trackbacks and pingbacks are open.