Controlling the brain with a click of a smartphone seems like the stuff of science fiction, but scientists from Korea and the United States have made it happen.
Their new device can control neural circuits using a tiny brain implant controlled by a smartphone. The researchers believe that the device can be used to speed up efforts to uncover brain diseases such as Parkinson’s, Alzheimer’s, addiction, depression, and even pain.
What is it?
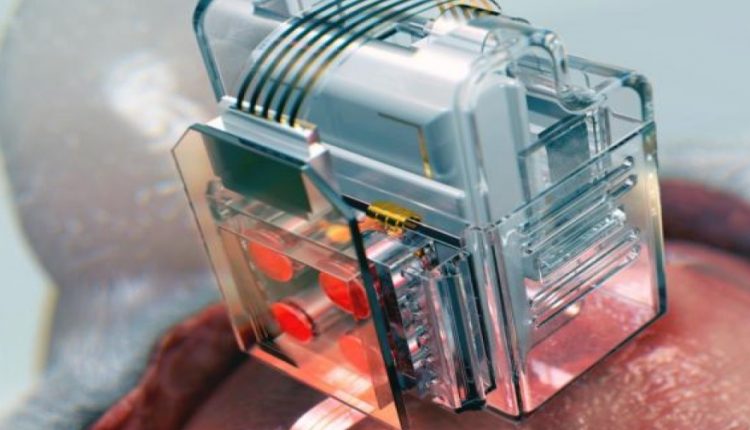
The device uses Lego-like replaceable drug cartridges and powerful Bluetooth low-energy to target specific neurons of interest using drug and light for prolonged periods.
“The wireless neural device enables chronic chemical and optical neuromodulation that has never been achieved before,” said Raza Qazi, a researcher with the Korea Advanced Institute of Science and Technology (KAIST) and University of Colorado Boulder.
According to Qazi, this technology overshadows conventional methods used by neuroscientists, which usually involve rigid metal tubes and optical fibers to deliver drugs and light. Apart from limiting the subject’s movement due to the physical connections with bulky equipment, their relatively rigid structure causes a lesion in soft brain tissue over time, therefore making them not suitable for long-term implantation. Though some efforts have been made partly mitigate adverse tissue response by incorporating soft probes and wireless platforms, the previous solutions were limited by their inability to deliver drugs for long periods of time as well as their bulky and complex control setups.
Wireless drug delivery
To achieve chronic wireless drug delivery, scientists had to solve the critical challenge of exhaustion and evaporation of drugs. Researchers from the Korea Advanced Institute of Science and Technology and the University of Washington in Seattle collaborated to invent a neural device with a replaceable drug cartridge, which could allow neuroscientists to study the same brain circuits for several months without worrying about running out of drugs.
These ‘plug-n-play’ drug cartridges were assembled into a brain implant for mice with a soft and ultrathin probe (thickness of a human hair), which consisted of microfluidic channels and tiny LEDs (smaller than a grain of salt), for unlimited drug doses and light delivery.
It is then controlled by a simple user interface on a smartphone to easily trigger any specific combination or precise sequencing of light and drug deliveries in any implanted target animal without need to be physically inside the laboratory. Using these wireless neural devices, researchers could also easily set up fully automated animal studies where the behavior of one animal could positively or negatively affect behavior in other animals by conditional triggering of light and/or drug delivery.
“This revolutionary device is the fruit of advanced electronics design and powerful micro and nanoscale engineering,” said Jae-Woong Jeong, a professor of electrical engineering at KAIST. “We are interested in further developing this technology to make a brain implant for clinical applications.”
The technology has the potential to help researchers by better dissecting neural circuit basis of behavior and determine how specific neuromodulators in the brain tune behavior in different ways. It has the potential to allow researchers to create new therapies for pain, addiction, and emotional disorders, according to the team.
Story via University of Washington Health Sciences
