New anode material stores 1.5x more electricity than graphite anode used in lithium-ion batteries
Various automobile companies are preparing to shift from internal combustion (IC) engine vehicles to electric vehicles (EVs). For EV costs to compete with those of IC engine vehicles, their batteries, which account for about 30% of their cost, must cost less.
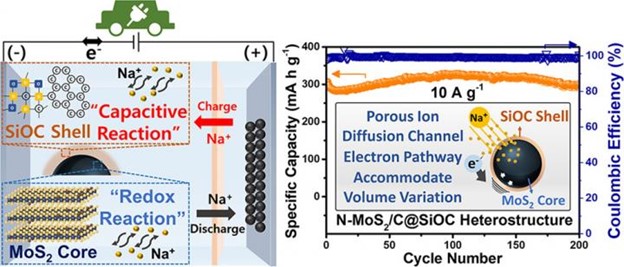
In response, the Korea Institute of Science and Technology (KIST) announced that Dr. Sang-Ok Kim’s team at the Center for Energy Storage Research developed a novel, high-performance, economical anode material for use in sodium-ion secondary batteries. The novel material stores 1.5x more electricity than a graphite anode used in commercial lithium-ion batteries and its performance does not degrade even after 200 cycles at very fast charging/discharging.
Sodium is over 500 times more abundant in the Earth’s crust than lithium and, in batteries, it is 40% cheaper. However, sodium ions are larger and, thus, cannot be stored as stably in graphite and silicon, which are widely used as anodes in such batteries, so the development of a novel, high-capacity anode material was necessary.
The KIST research team used molybdenum disulfide (MoS2). MoS2 can store a large amount of electricity but cannot be used because of its high electrical resistance and structural instability that occur during battery operation. The team overcame this problem by creating a ceramic nano-coating layer using silicone oil, a low-cost, eco-friendly material.
Original Release: Eureka Alert

Sodium is much much cheaper than lithium, and is also evenly distributed over more than 70% of the earth’s surface. It is heavier, and more of safety risk (catches fire in water), but if it can be got to work in batteries it could well be a way forward.
Sodium sulphur batteries were developed decades ago, but the drawback is that the sodium has to be kept molten are not very practicable for car use. OTOH maybe OK for rail.