Inspired by the chemistry of iron-hungry microorganisms like bacteria and fungi that secrete natural compounds known as “siderophores” to siphon essential nutrients from their hosts, researchers have created an artificial siderophore to improve the way materials select and bind uranium. The bio-inspired material provides an eco-friendly and cost-effective approach to recovering uranium from seawater.
A research team from the Department of Energy’s Oak Ridge and Lawrence Berkeley National Laboratories, the University of California, Berkeley, and the University of South Florida developed a material that selectively binds dissolved uranium with a low-cost polymer adsorbent. The adsorbent naturally selects uranium over other metals present in seawater and can easily be recycled for reuse.
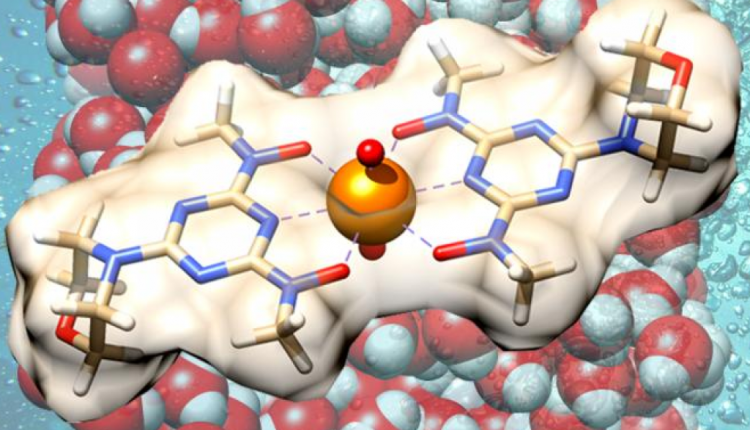
The team used computational and experimental methods to develop a novel functional group known as “H2BHT” (2,6-bis[hydroxy(methyl)amino]-4-morpholino-1,3,5-triazine) that preferentially selects uranyl ions, or water-soluble uranium, over competing metal ions from other elements in seawater, such as vanadium.
The fundamental discovery is backed by the promising performance of a proof-of-principle H2BHT polymer adsorbent. Uranyl ions are readily “adsorbed,” or bonded to the surface of the material’s fibers because of the unique chemistry of H2BHT. The prototype stands out among other synthetic materials for increasing the storage space for uranium, yielding a highly selective and recyclable material that recovers uranium more efficiently than previous methods.
With a practical recovery method, saltwater extraction offers a sustainable alternative to land-mining uranium that could sustain nuclear power production for millennia.
Uranium deposits are abundant and replenishable in seawater through the natural erosion of ore-containing rocks and soil. Despite dilute concentrations, approximately three milligrams of uranium per ton of seawater, the world’s oceans hold massive stores of the element totaling an estimated four billion tons—a thousand times more than all land sources combined. Scientists have been attempting to develop efficient uranium adsorbents to harness this potential resource since the 1960s, but amidoxime-based compounds had a much stronger attraction to vanadium than uranium, which posed a problem in mixed-metal water environments.
Amidoxime-based materials, the current front-runners for commercially available adsorbents, fill up more quickly with vanadium than uranium, and highly concentrated acidic solutions used to remove vanadium are costly and create caustic waste. The acid processing can damage material fibers, which limits their reuse, making commercial adoption cost-prohibitive.
Unlike vanadium-laden materials, the H2BHT polymer can be processed using mild basic solutions and recycled for extended reuse. The eco-friendly features also bring significant cost advantages to potential real-world applications.
