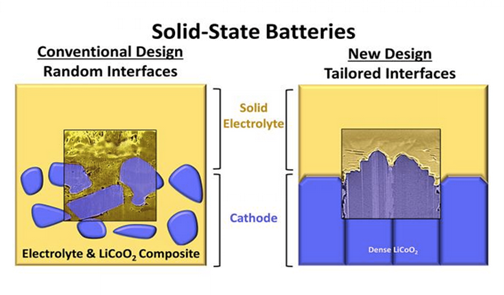
While it’s true that solid-state batteries pack substantial energy into a small space, their electrodes are not good at keeping in touch with their electrolytes. Similarly, liquid electrolytes reach every nook and cranny of an electrode to spark energy, but liquids take up space without storing energy and fail over time. Researchers are now putting solid electrolytes in touch with electrodes made of strategically arranged materials at the atomic level.
A recent study, led by University of Illinois Urbana-Champaign materials science and engineering professor Paul Braun, postdoctoral research associate Beniamin Zahiri, and Xerion Advanced Battery Corp. director of research and development John Cook, indicates that control over the atomic alignment of solid materials improves the cathode-solid electrolyte interface and stability in solid-state batteries. The results are published in the journal Nature Materials.
The researchers said an electrolyte’s stability controls how many charging and discharging cycles a battery handles before it starts to lose power. Scientists are now in a race to find the most stable electrolyte materials.
The team built electrodes containing sodium and lithium ions with specific atomic arrangements. They found correlations between battery performance and interface atomic arrangement in both the lithium- and sodium-based solid-state batteries and discovered that minimizing the interface surface area and controlling the electrodes’ atomic alignment is key to understanding the nature of interface instabilities and improving cell performance.
This level of control gave the researchers the information needed to run atomic simulations that they hypothesize will lead to even better electrolyte materials in the future, the researchers said.
Original Release: Eureka Alert
